

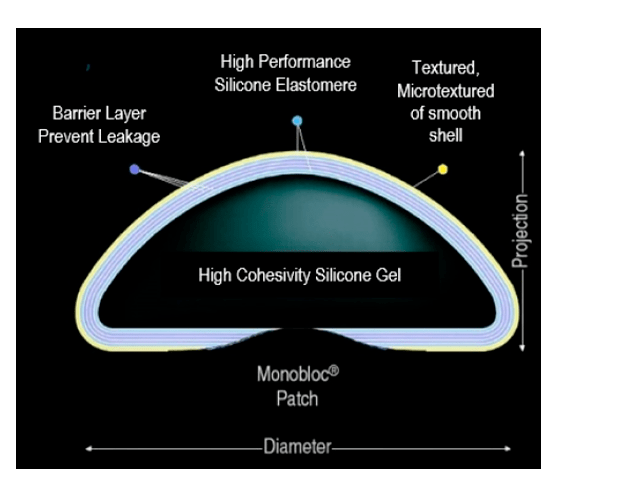
- 100% High Cohesive Biocompatible Silicone Gel.
- Regular wrap with 6 layers and very low break rate.
- Shape memory with consistency very similar to the mammary gland.
- 2 shapes: Round and Anatomical.
- 3 Surfaces (Smooth, Micro-textured and Textured) and 5 profiles (Low, High, Intermediate, Extra High and Ultra High).
Leading Innovation and Technology
Reliable and perfected products with a unique precision in immersion.
- Implants that meet the regulatory performance required by ISO 14607.
- Implants that received the CE mark by the notified organization Kiwa.
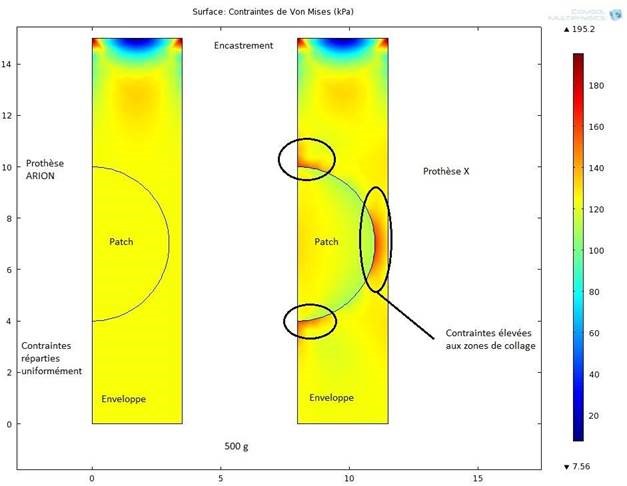
- Welding of the occlusion disc to the sheath, to ensure the lowest possible rupture rate.

LABORATOIRES ARION
History
In 1965 DOCTOR H.G. Arion, a plastic surgeon and physician, invented the world’s first inflatable prosthesis. Since then, millions of women have benefited from this innovation. To this inventive genius and passionate origins, it was necessary to add rigor, method, creativity and perfection. The sum of these values is found today in each implant and this makes Arion Laboratories a high-level reference for professionals in reconstructive and aesthetic surgery.
1994 – 2000
Laboratorios ARION was founded in 1994 by Dr. Ingrid Arion and its current president, Jean Claude Arion. Supported by Henri Gilbert Arion, surgeon who invented inflatable breast prostheses in 1965, Laboratorios ARION already has 30 years of research and clinical trials that allow it to be at the forefront of technology and safety in the field of implants. mammaries.
At that time, Arion Laboratories developed implants containing physiological saline or CMC hydrogel. The scientific adviser of the Arion laboratories estimated that the old generation silicone gel prostheses still did not offer sufficient safety to the patients (insufficient cohesion of the silicone gels, wrappers subjected to sweat, structural weakness at the level of the discs of occlusion). Otherwise, old-generation silicone gel prostheses are banned in the United States and were banned in France from 1995 to 2001.
In the years 2000 – 2010
Today and tomorrow…
Thanks to more than 40 years of experience validated by various international scientific publications, Arion Laboratories today proposes a range of “superior category” implants, designed according to the best available techniques in relation to with the safety of patients.
– With the Monobloc Hydrogel CMC range, Arion laboratories are currently the only ones* that can propose a biocompatible alternative to silicone gel implants.
*except with saline
High quality
Ethics and responsibility are key values in our opinion. Our requirement: to provide patients with products of absolute reliability and adapted to their expectations.
In terms of reliability, flexibility and safety, the Monobloc® breast implants from Laboratoires Arion respond to the demands of surgeons and their patients.
In addition, the wide range of implants proposed makes it possible to treat multiple cases, either through reconstructive or aesthetic surgery, and this while fully respecting the physiological characteristics of the patients.
In addition, los Laboratoires Arion is committed to patients with the Serenity Package. This is a true guarantee of satisfaction for them.
Results of the inspection of the AFSSAPS (French Agency for Sanitary Safety of Health Products)
In a desire for total transparency towards our surgeon clients and patients, our laboratory has decided to regularly communicate all the control tests, the biological and mechanical tests carried out on its manufacture.
In October 2011, mechanical tests performed on the shell of Monobloc breast implants showed results not only complying with the NF EN ISO14607 standard but also exceeding the requirements demanded.
